发表论文

Precise tailoring of pore chemistry is indispensable for efficient membrane gas separation, particularly for the challenging acetylene system. Here, a strategy called “anion substitution” is reported, to strengthen the interaction between anions and acetylene within the pores, for radically improving gas selectivity and permeability. The anions F− and OH− are infixed in iPAF-1 to replace the original Cl− ion. Their small anionic radii allow retention of the original high porosity of iPAF-1-Cl in iPAF-1-F and iPAF-1-OH. Highly basic F− and OH− confined in the pores attract acidic acetylene strongly and preferentially. Nanoparticles of iPAF-1 are processed to form mixed matrix membranes, represented by iPAF-1-OH/6FDA-ODA. The prepared membranes exhibit remarkable performance in separating acetylene from ethylene and ethane. Transplantation of porous and functional iPAF-1-OH into 6FDA-ODA significantly enhances both acetylene permeability (sevenfold) and permselectivity (fivefold) for acetylene over ethylene and ethane, which is crucial for membrane acetylene gas separation.
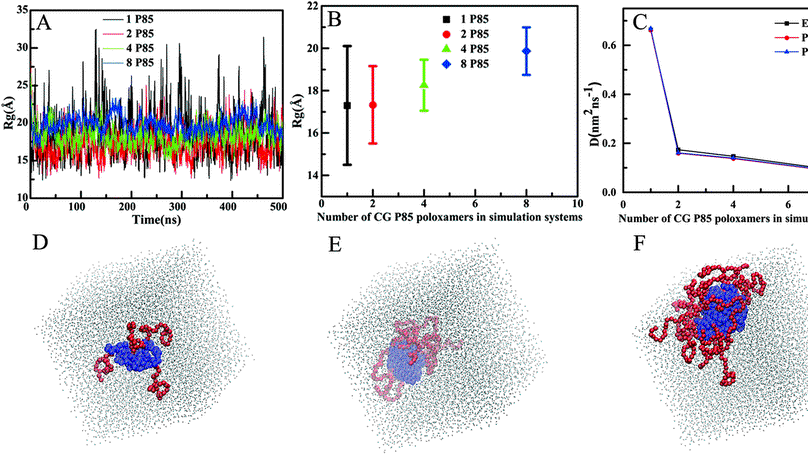
Polyethylene oxide (PEO) and poly(propylene oxide) (PPO), especially their tri-block copolymers PEO–PPO–PEO (poloxamers), have a broad range of applications in biotechnology and medical science. Understanding their specific interactions with biomembranes is the key to unveil the unique features of poloxamers either as membrane-healing or membrane pore-forming agents. Based on the coarse-graining convention of the MARTINI force field and the big multipole water (BMW) model, which has a three charged site topology and can reproduce the correct dipole moment of four-water clusters, we generated coarse-grained (CG) models with analytical and numerical potentials for PEO and PPO homopolymers and poloxamers in dilute solution. The effective bonded interaction potentials between CG beads were determined from the probability distributions of bond lengths, angles and dihedrals that are determined from atomistic simulations. The nonbonded interaction parameters were fine-tuned to reproduce the conformational properties of atomistic PEO and PPO homopolymers and poloxamers via extensive CG simulations of PEO and PPO homopolymers and poloxamers in a BMW water environment. The reported CG models provide a promising framework for a comprehensive understanding of the microstructural, conformational, and dynamic properties of poloxamers and their delicate interactions with other species in an explicit water environment.
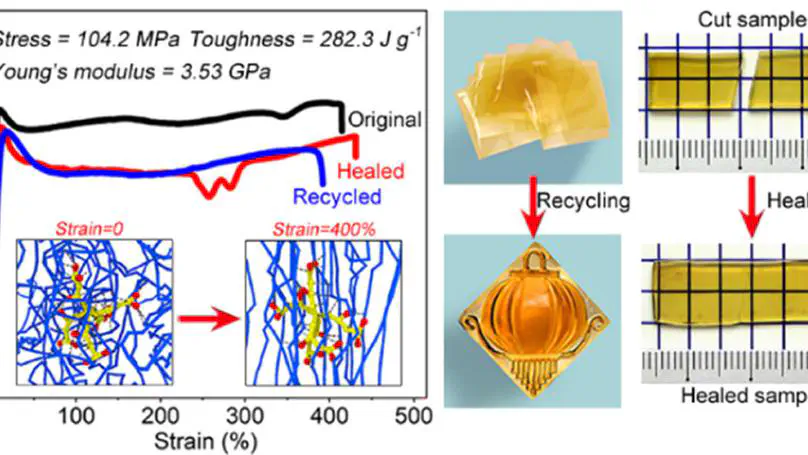
To build a sustainable society, it is of significant importance but highly challenging to develop remalleable, healable, and biodegradable polymeric materials with integrated high strength and high toughness. Here, we report a superstrong and ultratough sustainable supramolecular polymeric material with a toughness of ca. 282.3 J g–1 (395.2 MJ m–3) in combination with a tensile strength as high as ca. 104.2 MPa and a Young’s modulus of ca. 3.53 GPa. The toughness is even higher than that of the toughest spider silk (ca. 354 MJ m–3) ever found in the world, while the material also exhibits a superior tensile strength over most engineering plastics. This material is fabricated by topological confinement of the biodegradable linear polymer of poly(vinyl alcohol) (PVA) via the naturally occurring dendritic molecules of tannic acid (TA) based on high-density hydrogen bonds. Simply blending TA and PVA in aqueous solutions at acidic conditions leads to the formation of TA–PVA complexes as precipitates, which can be processed into dry TA–PVA composite products with desired shapes via the compression molding method. Compared to the conventional solution casting method for the fabrication of PVA-based thin films, the as-developed strategy allows large-scale production of bulk TA–PVA composites. The TA–PVA composites consist of interpenetrating three-dimensional supramolecular TA–PVA clusters. Such a structural feature, revealed by computational simulations, is crucial for the integrated superhigh strength and ultrahigh toughness of the material. The biodegradable TA–PVA composites are remalleable for multiple generations of recycling and healable after break, at room temperature, by the assistance of water to activate the reversibility of the hydrogen bonds. The TA–PVA composites show high promise as sustainable substitutes for conventional plastics because of their remalleability, healability, and biodegradability. The integrated superhigh strength and ultrahigh toughness of the TA–PVA composites ensure their high reliability and broad applicability.

Probing the transformation from crystalline form II with 11/3 helical structure to form I with 3/1 helical structure in PB-1 and its nanocomposites is of great importance in both scientific fields and commercial applications. The influence of lamellar thickness on the transformation from form II to form I in isotactic polybutylene-1 (PB-1)/carbon nanotube (CNT) nanocomposites was investigated by differential scanning calorimetry (DSC), polarized optical microscopy (POM) and small-angle X-ray scattering (SAXS) techniques. The crystallization kinetics of PB-1/CNT nanocomposites at different isothermal temperatures (Tc) indicates that CNTs significantly accelerate the nucleation and growth of form II, acting as heterogeneous nucleating agents. However, the influence of CNTs on the form II–I transformation strongly depends on the lamellar thickness obtained at different Tc, verified by the change in melting point (Tm) and the SAXS results for form I. The addition of CNTs accelerates the transformation and elevates the Tm of form I when the Tc is lower than ∼88 °C, and slows the transformation and slightly decreases the Tm when the Tc is higher than ∼88 °C. This is probably due to the fact that the incorporation of CNTs facilitates an increase in lamellar thickness of form II formed at lower Tc but decreases the lamellar thickness of form II formed at higher Tc. Our study illustrates that the lamellar thickness is one of the key points to determine the transformation from form II to form I in PB-1.

The rapid loss of active sulfur, because of a notorious effect of polysulfide shuttle, leads to a severe capacity fading in lithium–sulfur (Li–S) batteries. Although oxygen doping in cathodic materials is a promising strategy to enhance bonding interactions with lithium polysulfides, the origin of strong interactions remains to be poorly understood, because of a lack of consideration for the spatial arrangement of oxygen atoms affecting the overall performances. Here, we unveil the role of hydroxyl architecture on polysulfide trapping by systematically studying a series of cyclodextrin molecules that serve as a model platform, because of their well-defined structural uniqueness featuring abundant hydroxyl binding sites. We compare their trapping behaviors toward lithium polysulfides and thus correlate performance variations with their structural differences. Experimental findings coupled with computational modeling suggest that the asymmetrical arrangement of primary hydroxyl groups in β-CD determines the highest binding energies, relevant to the optimal capability in constraining polysulfide shuttle, and, in turn, contributes to remarkable improvements in the rate capacity and cycling performance. The identification of the role of hydroxyl architecture provides an atomic-scale explanation for the interaction between OH-group ordered interface and lithium polysulfides, and a new insight for the future design and engineering of interfaces in Li–S batteries.

The design and discovery of new two-dimensional materials with desired structures and properties are always one of the most fundamental goals in materials science. Here we present an atom-mimicking design concept to achieve direct self-assembly of two-dimensional low-coordinated open lattices using three-dimensional patchy particle systems. Besides honeycomb lattices, a new type of two-dimensional square-octagon lattice is obtained through rational design of the patch configuration of soft three-patch particles. However, unexpectedly the building blocks with thermodynamically favoured patch configuration cannot form square-octagon lattices in our simulations. We further reveal the kinetic mechanisms controlling the formation of the honeycomb and square-octagon lattices. The results indicate that the kinetically favoured intermediates play a critical role in determining the structure of obtained open lattices. This kinetics-controlled design principle provides a particularly effective and extendable framework to construct other novel open lattice structures.

Polymerization of monomers into two-dimensional covalent organic frameworks with precise porous structures exhibits desired catalytic, gas separation, and optoelectronic properties. However, the defects arising from covalent bonding in a polymerization process always result in amorphous films with small crystalline domains or polycrystalline powders. It is still a tremendous challenge to synthesize high-quality crystalline products, even single crystals with a large size over the micrometer scale. In this work, we propose a general strategy of building block design to reduce the defects during growth of two-dimensional covalent organic frameworks. We demonstrate that the building block with a hexagonal pore unit, i.e., a hexamer, could greatly decrease defects by directional uniform growth in polymerization, while monomer, dimer, and trimer building blocks form more defects due to linear growth. Our work provides a new strategy to construct superlarge single crystals in practical applications by combining building block design and growing dynamics control.

Compositional catalysts based on porous supports and incorporated catalytic nanoparticles have achieved great successes during the past decades. However, rational design of synergic catalysts and modulating the interactions between functional supports and catalytic sites are still far from being well developed. In this work, aiming at overcoming the difficulties of comprehensive screening of porous supports and correspondingly matched catalytic sites, a cationic porous aromatic framework as a capturing platform and polyoxometalate anions as conversion materials are separately designed, and their combination is modularly controlled. The resulting composites show higher catalytic activities than the corresponding conversion sites themselves. Notably, the resulting composites uncommonly exhibit increased surface area and enlarged pore openings after the incorporation of nanoparticles, and lead to the promotion of mass transfer within the porous supports. The emergence of a hierarchical structure with increased surface area induced by guest loading is desired in heterogeneous catalysis. The reciprocal modulation of both capture and conversion materials results in enhanced conversion and increased reaction rate, indicating the successful preparation of synergic catalysts by this separate design approach.

Synthetic vesicles of amphiphilic Janus dendrimers are known as dendrimersomes. The understanding of the conditions and formation mechanism of dendrimersomes is meaningful for further controlling the structures. Herein, the characteristics of the self-assembly of amphiphilic Janus dendrimer/water solutions into unilamellar and onion-like dendrimersomes are studied by molecular dynamics simulations via a spherical single-site Janus particle model. The model with two distinct surfaces, one hydrophobic side and another hydrophilic side, describes the amphiphilic nature of Janus dendrimers. By reducing the dendrimers with complex architectures to be simple Janus particles, we investigate the concentration-dependent self-assembled structures as well as the enthalpy-driven formation process of onion-like dendrimersomes, in contrast to the entropy-mediated self-assembly of amphiphilic flexible chains. Three typical equilibrium morphologies including linear micelles, lamellar structures and vesicles are found upon varying the Janus balance and dendrimer concentration. It is observed that the dendrimersomes consisting of the dendrimers with neglectable molecular configuration entropy become very stable, which agrees well with experimental observation. Specifically, different from many lipidsomes and polymersomes which can spontaneously merge, the size of dendrimersomes will not increase through mutual fusion once the well-defined onion-like structure is formed. Moreover, the discharge of water is achieved by water diffusion in our simulations, instead of in the “peeling-one-onion-layer-at-a-time” fashion. Our study combined with the previous ones using flexible chain models could depict a complete picture of dendrimersomes in favor of their applications in drug and gene delivery.