发表论文

The formation and transformation of defects in confined liquid crystals are fascinating fundamental problems in soft matter. Here, we use molecular dynamics (MD) simulations to study ellipsoidal liquid crystals (LCs) confined in a spherical cavity, which significantly affects the orientation and translation of LC molecules near the surface. The liquid-crystal droplet can present the isotropic to smectic-B phase transition through the smectic-A phase, as the number density of the LC molecules increases. We further find the change of LC structure from bipolar to watermelon-striped during the phase transition from smectic-A (SmA) to smectic-B (SmB) phases. Our results reveal the transition from bipolar defects to the inhomogeneous structures with the coexistence of nematic and smectic phases in smectic liquid-crystal droplets. We also study the influence of the sphere size in the range of 10σ0 ≤ Rsphere ≤ 50σ0 on the structural inhomogeneities. It shows a weak dependence on the sphere size. We further focus on how the structures can be affected by the interaction strength εGB–LJ. Interestingly, we find the watermelon-striped structure can be changed into a configuration with four defects at the vertices of a tetrahedron upon increasing the interaction strength. The liquid crystals at a strong interaction strength of εGB–LJ = 10.0ε0 show the two-dimensional nematic phase at the surface. We further present an explanation for the origin of the striped-pattern formation. Our results highlight the potential for using confinement to control these defects and their associated nanostructural heterogeneity.

Nafion, as the mostly used proton exchange membrane material in vanadium redox flow batteries (VRFBs), encounters serious vanadium permeation problems due to the large size difference between its anionic nanophase (3–5 nm) and cationic vanadium ions (∼0.6 nm). Bulk hybridization usually suppresses the vanadium permeation at the expense of proton conductivity since conventional additives tend to randomly agglomerate and damage the nanophase continuity from unsuitable sizes and intrinsic incompatibility. Here, we report the ionic-nanophase hybridization strategy of Nafion membranes by using fluorinated block copolymers (FBCs) and polyoxometalates (POMs) as supramolecular patching additives. The cooperative noncovalent interactions among Nafion, interfacial-active FBCs, and POMs can construct a 1 nm-shrunk ionic nanophase with abundant proton transport sites, preserved continuity, and efficient vanadium screeners, which leads to a comprehensive enhancement in proton conductivity, selectivity, and VRFB performance. These results demonstrate the intriguing potential of the supramolecular patching strategy in precisely tuning nanostructured electrolyte membranes for improved performance.

Soft fibres can be used to make smart textiles for use in energy, sensing and therapeutic applications. However, the fabrication of functional fibres is difficult compared with the fabrication of two-dimensional films and three-dimensional monoliths, and current methods typically require high temperatures, high volumes of solvents or complex systems. Here we report a spinning approach to fabricate functional fibres, which is based on spontaneous phase separation and is inspired by the silk-spinning processes of spiders. The silk-spinning process is mimicked by creating a spinning solution of polyacrylonitrile and silver ions, which forms an elastic supramolecular network with silver coordination complexes and in situ reduced silver nanoparticles. This approach, which operates at ambient pressure and temperature, can be used to make soft functional fibres that are mechanically stretchable (more than 500% strain), strong (more than 6 MPa) and electrically conductive (around 1.82 S m−1). To illustrate the capabilities of the technique, we use the fibres to create a sensing glove and a smart face mask.
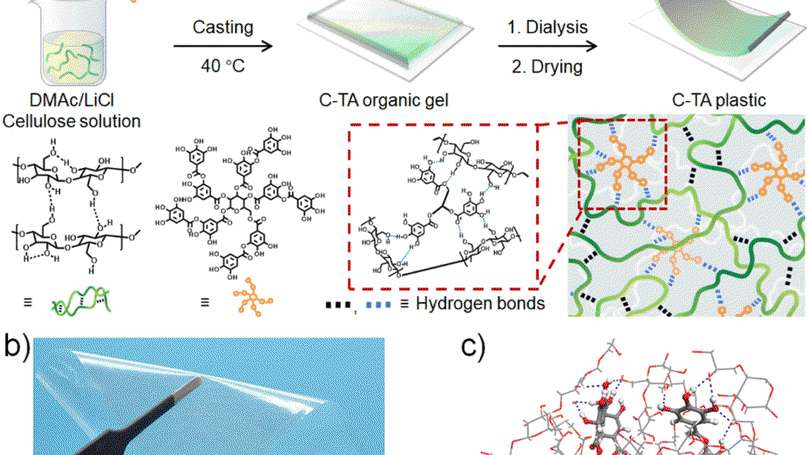
It is challenging to fabricate high-performance degradable plastics that simultaneously possess high mechanical strength, satisfactory water resistance and rapid degradation characteristics in natural environments using biomass resources. In this study, mechanically robust, water-resistant, biocompatible, and degradable plastics are fabricated through the complexation of regenerated cellulose and tannic acid (TA) followed by molding these complexes into desired shapes. The resulting plastic (denoted as C-TA) prepared with 15 wt% TA exhibits an ultrahigh fracture strength of ∼265 MPa and a toughness of ∼55.2 MJ m−3. An all-atom molecular dynamics simulation demonstrates that the introduction of dendritic TA molecules notably enhances the toughness of the C-TA plastic through the formation of TA-centered hydrogen-bond clusters. The C-TA plastic retains a fracture strength of ∼166 MPa and ∼98 MPa after being stored in environments with relative humidities of 80% and 100% for 7 days, respectively, indicating its excellent water resistance. The good water resistance and high mechanical strength of the C-TA plastic originate from the hydrophobic aromatic rings of its TA molecules and its TA-centered hydrogen-bond clusters which serve as cross-links and nanofillers to strengthen the plastic. The C-TA plastic can be fully degraded in soil into nontoxic species within 35 days.

Normally, defects in two-dimensional, circular, confined liquid crystals can be classified into four types based on the position of singularities formed by liquid crystal molecules, i.e., the singularities located inside the circle, at the boundary, outside the circle, and outside the circle at infinity. However, it is considered difficult for small aspect ratio liquid crystals to generate all these four types of defects. In this study, we use molecular dynamics simulation to investigate the defect formed in Gay–Berne, ellipsoidal liquid crystals, with small aspect ratios confined in a circular cavity. As expected, we only find two types of defects (inside the circle and at the boundary) in circular, confined, Gay–Berne ellipsoids under static conditions at various densities, aspect ratios, and interactions between the wall and liquid crystals. However, when introducing an external field to the system, four types of defects can be observed. With increasing the strength of the external field, the singularities in the circular, confined system change from the inside to the boundary and the outside, and the farthest position that the singularities can reach depends on the strength of the external field. We further introduce an alternating, triangular wave, external field to the system to check if we can observe the transformation of different defects within an oscillating period. We find that the position of the singularities greatly depends on the oscillating intensity and oscillating period. By changing the oscillating intensity and oscillating period of the external field, the defect types can be adjusted, and the transformation between different defects can be easily observed. This provides a feasible way to modulate liquid crystal defects and investigate the transformation between different defects.

In athermal all-polymer nanocomposites (all-PNCs), single-chain nanoparticles (SCNPs) are often considered to be well miscible with polymer matrixes due to their similarity in chemical compositions. However, internal cross-linking units of SCNPs must have different chemistries from the backbone monomers and, therefore, also from matrix chains. Here, we use large-scale molecular dynamics simulations to study the influence of solvent selectivity, particularly to internal cross-linkers in SCNPs, on dispersion state of SCNPs in all-PNC films upon solvent evaporation. Surprisingly, we find distinct dispersion states of SCNPs in drying films with different solvent selectivities. When the solvent is both good for cross-linkers and backbone/matrix monomers, SCNPs can be uniformly dispersed. However, when the backbone/matrix monomers have better solvophilicity than the cross-linkers and the solvophilicity of the latter is weak enough, we find segregation of SCNPs in surface regions. Such phenomena can be attributed to the intrinsic difference in the solvent density at an interface region from that in the bulk, which eventually results in the aggregation of SCNPs at the interface region where the solvent particles are much less than in the bulk. At the interface region, cross-linkers in the SCNPs will have less contact with the solvent and, therefore, less enthalpy penalty than being located in the bulk region of the film. The results demonstrate that solvent selectivity has a non-negligible effect on the structure of the composite film, which will inevitably have impacts on macroscopic properties of the film.

Fluorescence imaging in the second near-infrared (NIR-II) window is well suited for biological systems due to lower tissue scattering and micrometer-scale resolution at depths of millimeters. Unfortunately, none of the NIR-II fluorophores have clinical application due to low quantum yields, poor photostability, and longterm biosafety concerns. Herein, we develop NIR-II fluorescent nanoprobes based on the isolated cage monodisperse strategy, which significantly inhibits the π-π stacking interactions and restricts internal rotation of the arrested indocyanine green (ICG) fluorophores, which minimizes aggregation-caused quenching and enhances their NIR-II imaging performance. Unlike ICG’s imaging window of several minutes, the nanoformulations have reduced photobleaching and provide longer observation times. The increased imaging time allows high spatiotemporal resolution of blood vessels and lymphatic system. Benefiting from the high resolution and sensitivity of the nanoprobes, sentinel lymph nodes are accurately removed under fluorescence navigation. More intriguingly, the patency of lymphovenous anastomosis (LVA) is presented more intuitively by qualitative and quantitative NIR-II fluorescence signals, which will directly benefit the innovation and promotion of LVA.

Biological systems employ non-equilibrium self-assembly to create ordered nanoarchitectures with sophisticated functions. However, it is challenging to construct artificial non-equilibrium nanoassemblies due to lack of control over assembly dynamics and kinetics. Herein, we design a series of linear polymers with different side groups for further coordination-driven self-assembly based on shape-complementarity. Such a design introduces a main-chain confinement which effectively slows down the assembly process of side groups, thus allowing us to monitor the real-time evolution of lychee-like nanostructures. The function related to the non-equilibrium nature is further explored by performing photothermal conversion study. The ability to observe and capture non-equilibrium states in this supramolecular system will enhance our understanding of the thermodynamic and kinetic features as well as functions of living systems.

Advanced proton exchange membranes (PEMs) are highly desirable in emerging sustainable energy technology. However, the further improvement of commercial perfluorosulfonic acid PEMs represented by Nafion is hindered by the lack of precise modification strategy due to their chemical inertness and low compatibility. Here, we report the robust assembly of polyethylene glycol grafted polyoxometalate amphiphile (GSiW11) into the ionic nanophases of Nafion, which largely enhances the comprehensive performance of Nafion. GSiW11 can coassemble with Nafion through multiple supramolecular interactions and realize a stable immobilization. The incorporation of GSiW11 can increase the whole proton content in the system and induce the hydrated ionic nanophase to form a wide channel for proton transport; meanwhile, GSiW11 can reinforce the Nafion ionic nanophase by noncovalent cross-linking. Based on these synergistic effects, the hybrid PEMs show multiple enhancements in proton conductivity, tensile strength, and fuel cell power density, which are all superior to the pristine Nafion. This work demonstrates the intriguing advantage of molecular nanoclusters as supramolecular enhancers to develop high-performance electrolyte materials.

The mechanism of growth of one of the competitive topologies for covalent organic frameworks with constitutional isomers is poorly understood. Herein, we employ molecular dynamics to study the isoenergetic assembly of the rhombic square (sql) and Kagome lattice (kgm). The concentration, solvent conditions, and the reversibility of chemical reactions are considered by means of an Arrhenius two-state model to describe the reactions. High concentrations and poor solvent both result in sql, agreeing well with recent experiments. Moreover, the high reversibility of reactions gives rise to sql, while the low reversibility leads to kgm, suggesting a new way of regulating the topology. Our analyses support that the nucleation of isomers influenced by experimental conditions is responsible for the selection of topologies, which improves understanding of the control of topology. We also propose a strategy in which a two-step growth can be exploited to greatly improve the crystallinity of kgm.