发表论文
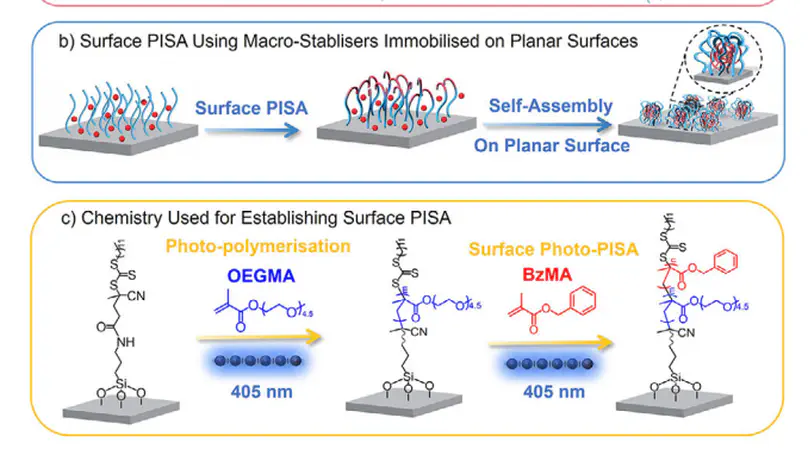
Polymerisation-induced self-assembly (PISA) has emerged as a highly efficient method for synthesising polymeric nanoparticles with diverse and well-defined morphologies for a range of applications. While extensive research has focused on solution-based PISA mediated by conformationally free macro-stabilisers, the process of PISA on planar surfaces using surface-tethered macro-stabilisers with constrained mobility, namely surface PISA, remains largely unexplored. Investigating this process is significant to further advance PISA technology and expand its applications. In this work, we explore surface PISA through both experimental and computational approaches, revealing key differences from conventional solution-based PISA. We also demonstrate that surface PISA offers an innovative approach for controlling surface topography and modulating material-bio interactions. Specifically, we showcase its versatile application in creating slippery liquid-infused porous surfaces (SLIPS) and encapsulating antibiotics, endowing material surfaces with enhanced antifouling and antimicrobial properties. We believe this work is a significant step forward for PISA technology and will create new opportunities for its broader applications.

The rapid synthesis of large-sized single-crystalline two-dimensional (2D) covalent organic frameworks (COFs) remains a formidable challenge with current synthetic techniques. A thorough microscopic comprehension of the dynamic processes that govern crystal growth from the perspective of defect formation/repair is imperative. Here, molecular dynamics simulations combined with a dynamic bond model are employed to track the real-time defect evolution in the growth of 2D COFs on surfaces. Our results indicate that defects at grain boundaries, caused by the random orientation of monomers, serve as critical barriers to crystal growth. During the growth process, defects evolve through multiple pathways, involving dynamic reorganization and selfrepair. We demonstrate that the interplay between activation energy and binding energy influences defect repair and, thereby, crystal growth and product morphology. Enhancing both forward and reverse reaction rates, achieved by reducing activation and binding energies within a narrow knife-blade parameter space, facilitates rapid defect repair and promotes single-crystal growth. These findings provide mechanistic insights into defect dynamics and inform the rational design of the reaction conditions for the synthesis of high-quality 2D COFs.

Supramolecular assemblies hold great promise for advanced chiral materials because of their structural diversity and dynamic features, but their low chiroptical activity limits practical applications. We report hierarchical supramolecular assemblies with giant chiroptical activity and mechanical attributes achieved through coassembly of achiral amphiphilic unimolecular micelles and chiral additives. Chiral fibrillar assemblies emerge from the nanostructured environment imposed by the micelles, driven by progressive chirality transfer through multiple hydrogen bonds between components. Integrating multifarious achiral luminescent molecules and nanocrystals into these assemblies leads to full-color circularly polarized luminescence–active materials with dissymmetry factors of ~10 −1 . A concentration-dependent chirality inversion is accessed through tailoring the coassembly kinetics. This strategy enables efficient red CPL, crucial for quantum and optical technologies. , Editor’s summary Hierarchical supramolecular materials assembled from achiral amphiphilic polymer micelles and chiral additives have high chiroptical activity across the visible spectrum. Kim et al . synthesized nanoscopic micelles with inner hydrophilic and outer hydrophobic blocks that hydrogen bonded to R- (or S-) mandelic acid to create nanobelts and ultimately chiral microfibers. Incorporating achiral fluorescent dyes and nanocrystals into these assemblies could produce circularly polarized emission with dissymmetry factors of about 0.1. —Phil Szuromi , INTRODUCTION Chirality is a fundamental characteristic of nature and living systems. Manifesting at multiple scales, both molecular and supramolecular chirality play essential roles across diverse disciplines, including physics, chemistry, biology, materials science, and nanotechnology. Polymer-based chiral supramolecular assemblies incorporating luminescent components have emerged as a promising class of circularly polarized luminescence (CPL)–active materials. These materials hold promise for applications in information encryption, imaging, display technologies, bioprobes, and spintronic and optoelectronic devices, while benefiting from the intrinsic advantages of polymers, including scalability, flexibility, and chemical diversity. However, conventional systems based on amphiphilic linear block copolymers (BCPs) struggle to achieve stable chiral structures with strong chiroptical activity, high quantum yield, and large luminescence dissymmetry factor ( g lum ) because of the dynamic instability of linear polymer assemblies, inefficient chirality transfer, and inadequate emitter protection. RATIONALE Amphiphilic star–like BCPs differ fundamentally from their linear analogs by forming unimolecular micelles with superior structural integrity. We hypothesized that coassembling achiral star–like BCPs with small chiral molecules could overcome the limitations of linear polymeric systems. Their structural stability and amphiphilicity make them compatible with a broad range of luminescent emitters through multiple noncovalent interactions, potentially enabling a general and efficient route to CPL generation across the visible spectrum. To explore this, we synthesized 21-arm star-like poly(acrylic acid)- block -polystyrene (PAA- b -PS) and coassembled it with enantiomeric mandelic acid [(R/S)-MA] through hydrogen bonding between MA and the hydrophilic PAA block, inducing chiral supramolecular structures. RESULTS Chiral supramolecular structures were constructed by thermal annealing–driven chiral coassembly of star-like PAA- b -PS and (R/S)-MA in dimethylformamide. The resulting assemblies exhibited microscale helical fibrillar morphology, enhanced mechanical attributes, and strong, stable circular dichroism signals spanning 200 to 500 nm with a dissymmetry factor of 0.039, indicating efficient chirality transfer from MA to the supramolecular assemblies. Time-dependent morphological and chiroptical characterization, together with coarse-grained molecular dynamics simulations, revealed a hierarchical organization. Initially, molecular chirality of MA was transferred to the PAA chains, inducing conformational chirality. Upon prolonged annealing, the complexes packed into kinetically trapped, belt-like secondary nanostructures, which subsequently reorganized into thermodynamically favored tertiary fibrillar microstructures with defined handedness. By leveraging the structural robustness and amphiphilicity of star-like BCPs, we demonstrated the versatility of coassemblies in generating CPL-active materials that emit strong CPL across the visible spectrum. We achieved high g lum (up to 0.11 in the blue region) by incorporating diverse achiral emitters, including hydrophilic and hydrophobic dyes, aggregation-caused quenching and aggregation-induced emission luminogens, and PS-ligated perovskite nanocrystals synthesized using star-like BCPs as nanoreactors. Through noncovalent interactions including hydrogen bonding, π–π stacking, and hydrophobic interactions, efficient chirality transfer to the emitters was achieved. These coassemblies exhibited enhanced quantum yields and prolonged emission lifetimes, which were attributed to reduced thermal degradation and aggregation within the chiral supramolecular framework. Furthermore, we demonstrated invertible chiroptical properties by modulating the assembly concentration and applying rapid solvent annealing using dimethylformamide-toluene mixtures. At high concentrations, densely packed rodlike structures were formed, exhibiting stronger chiroptical signals with opposite handedness. Coarse-grained molecular dynamics simulations and temperature-cycling experiments revealed that these inverted chiroptical states correspond to kinetically trapped, nonequilibrium structures. CONCLUSION We report the unique advantages of amphiphilic star-like BCPs in producing hierarchical chiral supramolecular assemblies that exhibit multiscale and tunable chirality, long-term stability, and enhanced mechanical properties. This strategy offers a universal and modular platform for developing full-color CPL-active materials with marked chiroptical performance. In particular, it enables strong red CPL, a longstanding challenge in many systems, highlighting its potential for emerging quantum technologies. A universal strategy for creating full-color, highly efficient CPL-active materials through hierarchical chiral supramolecular assemblies. Starting from achiral star–like PAA- b -PS block copolymers, hierarchical organization is driven by complexation with chiral R/S-MA, forming nanobelts and subsequently helical microfibers through chiral supramolecular assemblies. Incorporation of diverse emitters ranging from hydrophilic and hydrophobic dyes to aggregation-caused quenching and aggregation-induced emission luminogens, as well as inorganic perovskite nanocrystals, into the chiral assemblies enables stable, full-color, tunable CPLs with high efficiency.

Polymerization-induced self-assembly (PISA) offers a versatile platform for designing polymeric nanoparticles. Amphiphilic gradient copolymers, characterized by a gradual transition from hydrophilic to hydrophobic segments, exhibit reduced interfacial tension and enhanced stimulus responsiveness. However, the interplay between polymerization and self-assembly in PISA, influenced by the monomer feed ratio and reactivity, remains ambiguous. Herein, we employ coarse-grained simulations to investigate the role of the effective polymerization bias between monomers. Our results reveal that the relative monomer reactivity plays a key role in determining both the copolymer sequence and the vesicle formation pathway. At low reactivity differences, comparable monomer reactivities facilitate a cooperative polymerization-assembly process that produces numerous small spherical assemblies, which subsequently merge and reorganize into vesicles. In contrast, high reactivity asymmetry favors the formation of anisotropic worm-like micelles that progressively fuse, bend, and enclose into vesicular structures. Microstructural analysis further shows that gradient copolymer vesicles possess internal cavities larger than those formed from block copolymers. These insights provide guidance for tailoring vesicle formation pathways and fine-tuning microstructures for potential applications in drug delivery and materials science.

Electrolyte materials with responsive conductive properties are highly desired in electronic and sensing technologies, which rely on the construction of ion transport channels that combine orderliness with dynamic adjustability. However, achieving such structures remains a significant challenge. In this study, we fabricate a lamellar liquid crystal electrolyte enabling deformation-responsive proton conduction. Polyoxometalate nanoclusters (POMs) and zwitterionic molecules are utilized to construct the electrolytes through a supramolecular eutectic strategy. By balancing electrostatic and hydrogen-bonding interactions, zwitterionic molecules direct lamellar POM assembly while softening the system via hydrogen-bond-induced eutectic effect. This approach ultimately results in a POM-based room-temperature liquid crystal with a unique lamellar superlattice structure. Notably, the integration of proton-conductive POMs with dynamically responsive liquid crystal channels enables a highly sensitive change in proton conductivity under deformation. These findings expand the potential applications of liquid crystal systems and provide valuable insights for the development of responsive electrolyte materials.

The study of intrinsically disordered proteins (IDPs) and their role in biomolecular condensate formation has become a critical area of research, offering insights into fundamental biological processes and therapeutic development. Here, we present IPAMD (Intrinsically disordered Protein Aggregation Molecular Dynamics), a plugin-based software designed to simulate the formation dynamics of biomolecular condensates of IDPs. IPAMD provides a modular, efficient, and customizable simulation platform specifically designed for biomolecular condensate studies. It incorporates advanced force fields, such as HPS-based and Mpipi models, and employs optimization techniques for large-scale simulations. The software features a user-friendly interface and supports batch processing, making it accessible to researchers with varying computational expertise. Benchmarking and case studies demonstrate the ability of IPAMD to accurately simulate and analyze condensate structures and properties.

PyGAMD (Python GPU-accelerated molecular dynamics software) is a molecular simulation platform developed from scratch. It is designed for soft matter, especially for polymer by integrating coarse-grained/multi-scale models, methods, and force fields. It essentially includes an interpreter of molecular dynamics (MD) which supports secondary programming so that users can write their own functions by themselves, such as analytical potential forms for nonbonded, bond, angle, and dihedral interactions in an easy way, greatly extending the flexibility of MD simulations. The interpreter is written by pure Python language, making it easy to be modified and further developed. Some built-in libraries written by other languages that have been compiled for Python are added into PyGAMD to extend it’s features, including configuration initialization, property analysis, etc. Machine learning force fields that are trained by DeePMD-kit are supported by PyGAMD for conveniently implementing multi-scale modeling and simulations. By designing an advanced framework of software, graphics processing unit-acceleration achieved by the Numba library of Python and compute unified device architecture reaches a high computing efficiency.

Artificial skin is essential for bionic robotics, facilitating human skin–like functions such as sensation, communication, and protection. However, replicating a skin-matched all-in-one material with excellent mechanical properties, self-healing, adhesion, and multimodal sensing remains a challenge. Herein, we developed a multifunctional hydrogel by establishing a consolidated organic/metal bismuth ion architecture (COMBIA). Benefiting from hierarchical reversible noncovalent interactions, the COMBIA hydrogel exhibits an optimal combination of mechanical and functional properties, particularly its integrated mechanical properties, including unprecedented stretchability, fracture toughness, and resilience. Furthermore, these hydrogels demonstrate superior conductivity, optical transparency, freezing tolerance, adhesion capability, and spontaneous mechanical and electrical self-healing. These unified functions render our hydrogel exceptional properties such as shape adaptability, skin-like perception, and energy harvesting capabilities. To demonstrate its potential applications, an artificial skin using our COMBIA hydrogel was configured for stimulus signal recording, which, as a promising soft electronics platform, could be used for next-generation human-machine interfaces.

Developing artificial nanomaterial systems that can convert external stimuli to achieve nanoscale self-sustainable motion (for example, self-rotation), and simultaneously integrate and deploy the spatial localization of multiple active sites to unravel the intraparticle diffusion patterns of molecules, is of great importance for green synthetic chemistry. Here we show a paddle-like self-stirring mesoporous silica nanoreactor system with separated chambers and controllable proximity of active sites. The nanoreactors are designed by encapsulating magnetic Fe3O4 (~20 nm) in the first chamber, and meantime, Au and Pd nanocrystals are spatially isolated in different domains. Such a nanoreactor generates nanoscale rotation under the rotating magnetic fields and exhibits an order of magnitude activity enhancement in the cascade synthesis of 5,6-dimethylphenanthridinium (96.4% selectivity) compared with conventional macro-stirring. Meanwhile, we quantitatively uncovered the rotation-induced enhancement in sequential and reverse transfer of reactive intermediates, consequently revealing the relevance of self-rotation and proximity effects in controlling the catalytic performance.

Compared with current lipid nanoparticle delivery systems, a new drug delivery system that can simultaneously achieve high stability toward temperature and time, and controllable release of drugs will be smart and next-generation. However, designing such systems for the complex human body environment remains a daunting challenge. Herein, we use highly stable multilayer dendrimersomes as a model to study the mechanism of controlled release of drugs through stimulus-response by dissipative particle dynamics simulations. The results show that when the dendrimersomes remain intact, the release of encapsulated hydrophilic, hydrophobic, and neutral drugs is minimal. Once the amphiphilic dendrimers in the dendrimersomes are decomposed beyond a threshold by cleaving the linkers connecting hydrophobic and hydrophilic segments, which can be achieved by exogenous perturbations, a significant or complete release of the drugs occurs. The introduction of liquid flow will remarkably enhance the release capability of drugs in decomposed dendrimersomes. These insights into the controlled release of drugs at the microscopic level offer helpful guidance for the development of advanced drug delivery vehicles.