摘要
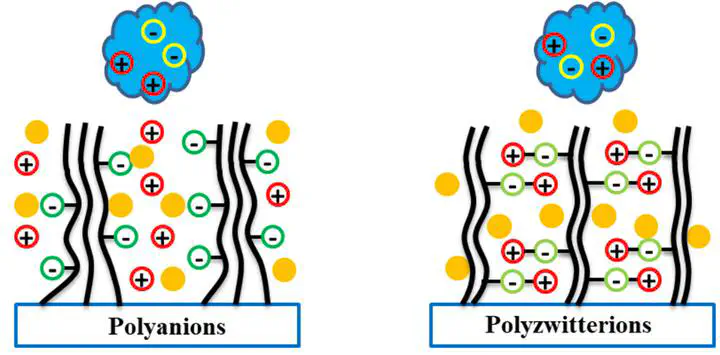
Surfaces grafted with polyelectrolyte chains for excellent performance in protein antifouling are highly desired in many applications, such as biomedical implants and devices. In general, the adsorbing/resisting behaviors of proteins can be mainly attributed to the electrostatic interactions that are associated with the charge properties of proteins and polyelectrolytes. By coarse-grained molecular dynamics simulations, we examined the self-assembled structures of polyanion and polyzwitterion brushes as well as the interactions on negatively and positively charged proteins. We found that in addition to charges, the structural polarization induced by self-assembly with a certain charge distribution shows significant influences on protein behavior. The large-scale dipole–dipole interactions between brushes and proteins can dominate the behavior of proteins on the brushes under certain circumstances. To ensure simulation accuracy, we compared two models and found a polar Martini model that explicitly treats electrostatic interactions as long-ranged ones, giving a more reasonable structural description compared with the normal Martini model that truncates electrostatic interactions.