Hierarchical chiral supramolecular assemblies with strong and invertible chiroptical properties
摘要
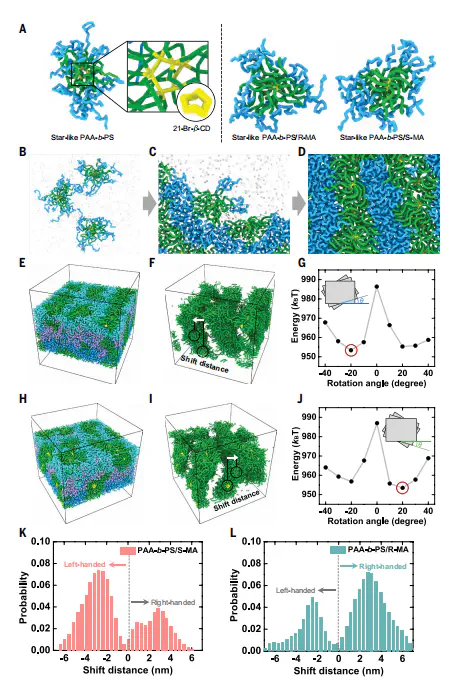
Supramolecular assemblies hold great promise for advanced chiral materials because of their structural diversity and dynamic features, but their low chiroptical activity limits practical applications. We report hierarchical supramolecular assemblies with giant chiroptical activity and mechanical attributes achieved through coassembly of achiral amphiphilic unimolecular micelles and chiral additives. Chiral fibrillar assemblies emerge from the nanostructured environment imposed by the micelles, driven by progressive chirality transfer through multiple hydrogen bonds between components. Integrating multifarious achiral luminescent molecules and nanocrystals into these assemblies leads to full-color circularly polarized luminescence–active materials with dissymmetry factors of ~10 −1 . A concentration-dependent chirality inversion is accessed through tailoring the coassembly kinetics. This strategy enables efficient red CPL, crucial for quantum and optical technologies. , Editor’s summary Hierarchical supramolecular materials assembled from achiral amphiphilic polymer micelles and chiral additives have high chiroptical activity across the visible spectrum. Kim et al . synthesized nanoscopic micelles with inner hydrophilic and outer hydrophobic blocks that hydrogen bonded to R- (or S-) mandelic acid to create nanobelts and ultimately chiral microfibers. Incorporating achiral fluorescent dyes and nanocrystals into these assemblies could produce circularly polarized emission with dissymmetry factors of about 0.1. —Phil Szuromi , INTRODUCTION Chirality is a fundamental characteristic of nature and living systems. Manifesting at multiple scales, both molecular and supramolecular chirality play essential roles across diverse disciplines, including physics, chemistry, biology, materials science, and nanotechnology. Polymer-based chiral supramolecular assemblies incorporating luminescent components have emerged as a promising class of circularly polarized luminescence (CPL)–active materials. These materials hold promise for applications in information encryption, imaging, display technologies, bioprobes, and spintronic and optoelectronic devices, while benefiting from the intrinsic advantages of polymers, including scalability, flexibility, and chemical diversity. However, conventional systems based on amphiphilic linear block copolymers (BCPs) struggle to achieve stable chiral structures with strong chiroptical activity, high quantum yield, and large luminescence dissymmetry factor ( g lum ) because of the dynamic instability of linear polymer assemblies, inefficient chirality transfer, and inadequate emitter protection. RATIONALE Amphiphilic star–like BCPs differ fundamentally from their linear analogs by forming unimolecular micelles with superior structural integrity. We hypothesized that coassembling achiral star–like BCPs with small chiral molecules could overcome the limitations of linear polymeric systems. Their structural stability and amphiphilicity make them compatible with a broad range of luminescent emitters through multiple noncovalent interactions, potentially enabling a general and efficient route to CPL generation across the visible spectrum. To explore this, we synthesized 21-arm star-like poly(acrylic acid)- block -polystyrene (PAA- b -PS) and coassembled it with enantiomeric mandelic acid [(R/S)-MA] through hydrogen bonding between MA and the hydrophilic PAA block, inducing chiral supramolecular structures. RESULTS Chiral supramolecular structures were constructed by thermal annealing–driven chiral coassembly of star-like PAA- b -PS and (R/S)-MA in dimethylformamide. The resulting assemblies exhibited microscale helical fibrillar morphology, enhanced mechanical attributes, and strong, stable circular dichroism signals spanning 200 to 500 nm with a dissymmetry factor of 0.039, indicating efficient chirality transfer from MA to the supramolecular assemblies. Time-dependent morphological and chiroptical characterization, together with coarse-grained molecular dynamics simulations, revealed a hierarchical organization. Initially, molecular chirality of MA was transferred to the PAA chains, inducing conformational chirality. Upon prolonged annealing, the complexes packed into kinetically trapped, belt-like secondary nanostructures, which subsequently reorganized into thermodynamically favored tertiary fibrillar microstructures with defined handedness. By leveraging the structural robustness and amphiphilicity of star-like BCPs, we demonstrated the versatility of coassemblies in generating CPL-active materials that emit strong CPL across the visible spectrum. We achieved high g lum (up to 0.11 in the blue region) by incorporating diverse achiral emitters, including hydrophilic and hydrophobic dyes, aggregation-caused quenching and aggregation-induced emission luminogens, and PS-ligated perovskite nanocrystals synthesized using star-like BCPs as nanoreactors. Through noncovalent interactions including hydrogen bonding, π–π stacking, and hydrophobic interactions, efficient chirality transfer to the emitters was achieved. These coassemblies exhibited enhanced quantum yields and prolonged emission lifetimes, which were attributed to reduced thermal degradation and aggregation within the chiral supramolecular framework. Furthermore, we demonstrated invertible chiroptical properties by modulating the assembly concentration and applying rapid solvent annealing using dimethylformamide-toluene mixtures. At high concentrations, densely packed rodlike structures were formed, exhibiting stronger chiroptical signals with opposite handedness. Coarse-grained molecular dynamics simulations and temperature-cycling experiments revealed that these inverted chiroptical states correspond to kinetically trapped, nonequilibrium structures. CONCLUSION We report the unique advantages of amphiphilic star-like BCPs in producing hierarchical chiral supramolecular assemblies that exhibit multiscale and tunable chirality, long-term stability, and enhanced mechanical properties. This strategy offers a universal and modular platform for developing full-color CPL-active materials with marked chiroptical performance. In particular, it enables strong red CPL, a longstanding challenge in many systems, highlighting its potential for emerging quantum technologies. A universal strategy for creating full-color, highly efficient CPL-active materials through hierarchical chiral supramolecular assemblies. Starting from achiral star–like PAA- b -PS block copolymers, hierarchical organization is driven by complexation with chiral R/S-MA, forming nanobelts and subsequently helical microfibers through chiral supramolecular assemblies. Incorporation of diverse emitters ranging from hydrophilic and hydrophobic dyes to aggregation-caused quenching and aggregation-induced emission luminogens, as well as inorganic perovskite nanocrystals, into the chiral assemblies enables stable, full-color, tunable CPLs with high efficiency.